Cell Lysates
Universal Filter Plate for Bacterial, Insect, and Human Cell Lysates
An Apparatus for high-throughput filtration of
cell lysates, 96-well plate: The clarification of cell
requires separation of cell debris, colloids, insoluble
precipitates, aggregates, and other contaminants before the target molecule (proteins) is isolated and characterized.
Orochem’s filtration plate is a depth microfiltration system that consists of a filter with an open-pore structure to remove cells and cell debris; and
a filter–aid material with a tighter pore structure to remove colloidal
matter simultaneously to deliver particulate-free feed for down-stream processes.
Formats
High-throughput 96-well plate format Formats Small scale single-use depth filtration systems for large sample volumes (up to 2 liters)
Advantages
Efficient and reproducible clarification of cell lysates
Fast and high-throughput process. Reduce the processing time to 5 min. Good protein recovery
Good sample volume recovery
Results comparable or superior to high-speed centrifugation
Procedures:
A. Additional Materials RequiredVariable-speed bench-top centrifuge with rotor and appropriate carriers capable of handling stacked plates at 1000 x g
B. General Procedure for Lysate Filtration Using 96-well plate by Centrifugation
- Perform lysate clarification at room temperature or at 4°C.
- Place the filter plate on top of a collection plate.
- Apply up to 800 µL of cell lysate to each well.
- Place the assembly into a centrifuge with appropriate carriers capable of handling stacked plates,balance the centrifuge with appropriate plates, and centrifuge at 1000 x g for 2 minutes to collectthe clarified cell lysate.
Cell Lysate Filter
Experimental Data:
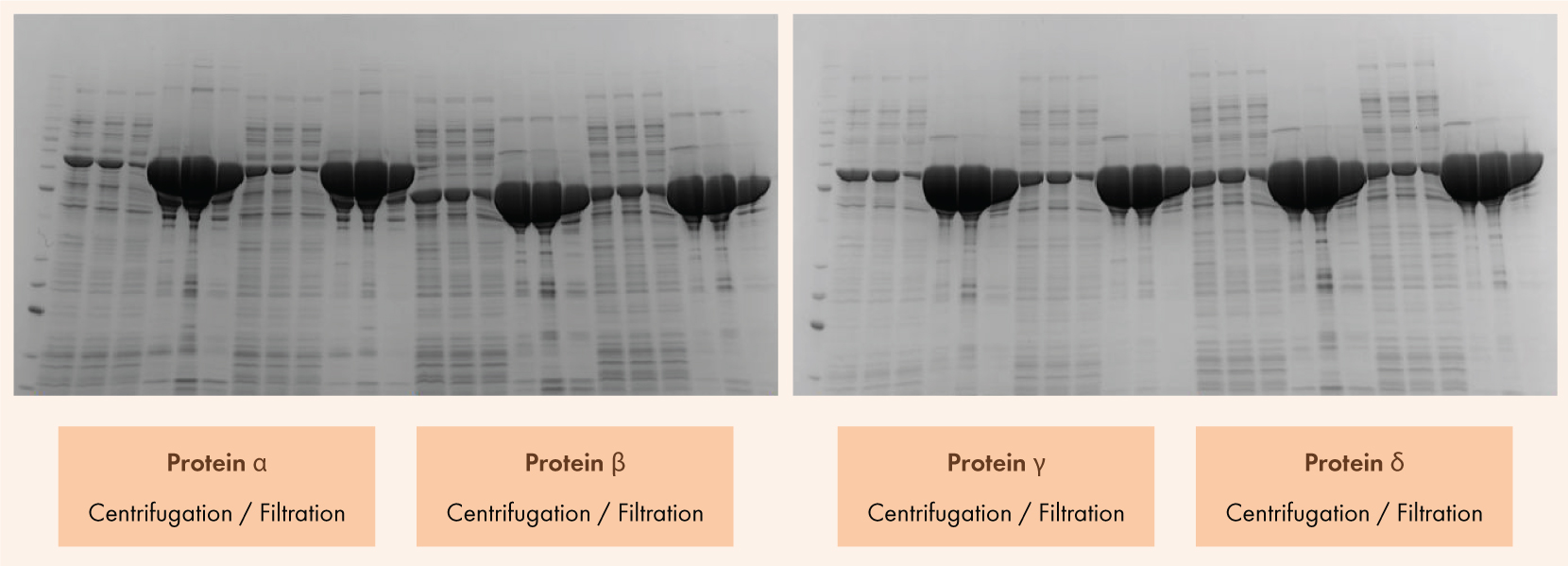
Figure 1.
SDS Page Results of E. Coli cell lysate by centrifugation and Cell Lysate Filter Plate. For every pair of samples, the left side was clarified using the centrifuge method without Cell Lysate Filter Plate and the right side was clarified using the filtration method. E.Coli growths were harvested by spinning at 4,000 RPM for 20 minutes at 4 °C. Pellets were resuspended in buffer 20/300/1 1:200 PI and lysed using a microfluidizer (LM20), 2 passes each, and the lysate was clarified using the centrifuge method 10,000 x g for 15 minutes and the filtration method 1,000 x g 2 min. Samples (440 µL) were purified using the PhyNexus MEA system. T= Total protein, L=Load protein (soluble), F=Flow through and
1,2,3= Elutions

 ”
”