- Catalog#
- Description
-

Sample Preparation Perspectives (1)
Sample Preparation Perspectives
Overview
The sample preparation step in an analytical process typically consists of an extraction procedure that results in the isolation and enrichment of components of interest from a sample matrix. Extraction can vary in degree of selectivity, speed, and convenience and depends not only on the approach and conditions used but on the geometric configurations of the extraction phase. Increased interest in sample preparation research has been generated by the introduction of nontraditional extraction technologies. These technologies address the need for reduction of solvent use, automation, and miniaturization and ultimately lead to on-site in situ and in vivo implementation. These extraction approaches are frequently easier to operate but provide optimization challenges. More fundamental knowledge is required by an analytical chemist not only about equilibrium conditions but, more importantly, about the kinetics of mass transfer in the extraction systems. Optimization of this extraction process enhances overall analysis. Proper design of the extraction devices and procedures facilitates convenient on-site implementation, integration with sampling, and separation/quantification, automation, or both. The key to rational choice, optimization, and design is an understanding of the fundamental principles governing mass transfer of analytes in multiphase systems. The objective of this perspective is to summarize the fundamental aspects of sample preparation and anticipate future developments and research needs.[accordions id="19333"] -

Filtration Sample Preparation (17)
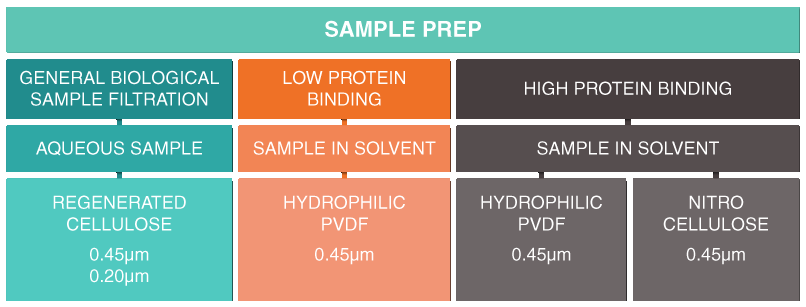
Filtration Sample Preparation
"Orochem’s vast selection of filtration plates allow preliminary mode of sample preparation of complex matrices. Often pre-filtration of plasma, serum and other biological fluids may be sufficient for sample preparation for chromatographic methods. Recommended for Dilute and Shoot and for sample clean up prior to HPLC and Micro arraying.

Protein Crash Plate and Protein Precipitation Plate (10)
Protein Crash Plate and Protein Precipitation
Protein precipitation is a common protocol for rapid sample clean-up and extraction of pharmaceuticals from blood samples (whole blood, plasma and serum) during drug discovery. It also allows the disruption of drug binding to proteins. Blood samples from various species such as dogs, rats, mice, and humans show variations in their compositions, but often only a single bioanalytical method covers all these species. Protein precipitation denatures the protein and destroys its drug binding ability, depending on the binding mechanism. [accordions id="19335"]
[accordions id="19335"]
Phospholipid Depletion (3)
Phospholipid Depletion
Purity is a unique phospholipid depletion device which integrates filtration with targeted removal of phospholipids and proteins in plasma/serum. The phospholipid retention mechanism is based on highly selective Lewis acid-base interaction between the ions (functionally bonded to the sorbent) and the phosphate moiety consistent with all phospholipids.Features- Both in column and 96-well plate formats
- Two different types of filters available, 0.45 and 0.20 micron.

MatriKleen Extraction (1)
MatriKleen Extraction Plate
MatriKleen Extraction Plate is a new generation sample preparation device which effectively removes most unwanted interferences from biological samples and eliminates ion suppression for mass spectrometry analysis.Features
- In both 3 cc cartridge and 2 cc/well 96-well plate format, larger cartridge format is also available
- Designed for plasma, serum, and blood samples

Benefits
- Universal extraction tool for:
- Polar and non-polar chemicals
- Acidic and basic analytes
- Quick and simple high throughput procedure
- Total process time
- Eliminates matrix effect from phospholipids and proteins
- High recovery and reproducibility for most drug compounds
Example Procedures
- Place MatriKleen extraction plate and collection plate on top of positive pressure processor (or vacuum manifold).
- Add 400 μL of methanol-acetonitrile mixture (1/1, v/v) to MatriKleen plate first, followed by 100 μL sample (spiked with target analyte).
- Wait about 5 minutes. Apply positive pressure or vacuum, adjust pressure so the sample flows through drop by drop.
- When all of solution is out, increase pressure to dry the plate for about 30 seconds.
- The eluate in the collection plate is ready to be analyzed.

Supported Liquid-Liquid Extraction (5)
Supported Liquid-Liquid Extraction
Traditional liquid-liquid extraction(LLE) utilizes large volumes of solvents that are often hazardous from an environmental perspective and the process is tedious and time consuming. During the past decade, this technique has undergone a spectacular transformation in the context of high-throughput screening with the introduction of miniaturized protocols along with revolutionary membrane-based and solid support based technologies. The simplest technique is the use of the 96-well plate format in conjunction with a liquid handling robotic system; it follows the same principle as bulk scale LLE. However, immobilization of the aqueous plasma sample on an inert solid support medium packed in a cartridge or in the individual wells of a 96-well plate and percolating a water immiscible organic solvent to extract the analyte from this medium evoked significant enthusiasm from the pharmaceutical industry. Orochem Aquamatrix cartridges and plates are packed with flux calcined diatomaceous earth for high capacity retention of analytes.
Features and Applications
- Packed with chemically inert material, stable in the pH range 1-13.
- Plates compatible with robotic liquid handlers for automated extraction.
- Allows high through put alternative to traditional liquid-liquid extraction techniques.
- Eliminates steps like mixing, centrifugation and organic phase separation.
- Provides support, greater surface area and accelerated solvent extraction.
- No pre-treatment of the bed is necessary.

Solid Phase Extraction (462)
Solid Phase Extraction
"SPE is a method of sample preparation that concentrates and purifies analytes from solution by sorption onto a sorbent contained in either a disposable solid phase cartridge or a 96-well plate, followed by elution of the analyte with a solvent and reconstitution into a mobile phase that is appropriate for the instrumental method used for analysis” (E.M.Thurman and M.S.Mills, Solid-Phase Extraction – Principles and Practice, John Wiley, New York, 1998) [accordions id='19320']
[accordions id='19320']
Vitamin D Metabolites Extraction Plate (1)
Vitamin D metabolites extraction plate
Orochem newly patented Vitamin D Metabolites Extraction plate is specifically designed for vitamin D metabolites analysis in clinical laboratory. The Vitamin D Metabolites extract plate provides for the extraction from human plasma or serum samples comprising vitamin D metabolites such as 1,25-dihydroxy vitamin D3 and D2, 25-hydroxy vitamin D2, and D3 from protein binding, removal of protein and phospholipids, and isolation of the metabolites using a combination of ion-exchange and Lewis acid mechanisms without the requirement to acidify the samples. The recommended method and the plate provides higher recoveries of vitamin D metabolites than existing phospholipid depletion plate techniques. Accurate quantification of 1,25-dihydroxy vitamin D and 25-hydroxy vitamin D metabolites is useful in differential diagnosis of vitamin D-related diseases and for monitoring vitamin D therapy in patients with chronic renal disease.Features- In 2 cc/well 96-well plate formate. Cartridge format is also available
- Recommended protocol
- 96-well format, 4 steps, totally less than 20 minutes per plate
- Simple, minimum training required
- Consistent and high recovery
- No matrix effect

QuEChERS (24)
OroQ QuEChERS Dispersive Products
Overview
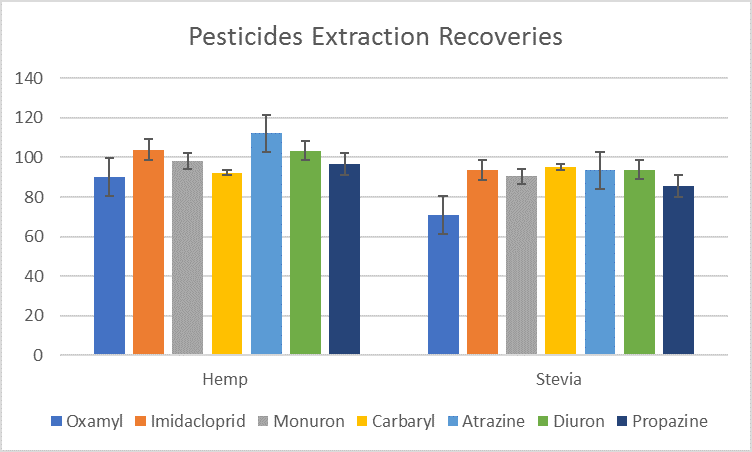
QuEChERS methods are developed for multi- pesticides residue analysis in foods. They are quick, easy, cheap, effective, rugged and safe. Orochem provides ready-to use QuEChERS dispersive SPE tubes with different types of sorbents and buffers in different sizes of centrifuge tubes. All sorbents and buffers are accurately measured and premixed, and ready for use. Orochem’s dispersive QuEChERS products are designed for either AOAC methods or for special applications, and are used to remove water, pigments, wax, fatty acids, and achieve high recovery.- For AOAC method 2007.01 general fruits and vegetables
- For AOAC method 2007.01 fruits and vegetables with fat and wax
- For AOAC method 2007.01 pigmented fruits and vegetables
- Orochem specialty, for tea and green leaves, complete pigment removal
- Orochem specialty, for tea and green leaves, high recovery
- Orochem specialty, for fruits including pigmented
- Orochem specialty, for stevia
- Orochem specialty, for hemp
Features:
- Pack directly in centrifuge tubes for convenience
- Available in 2 and 15 mL centrifuge tubes.
- Applicable for pesticide and food ingredients analysis
- Fit for GC, HPLC, and Ambient Mass spectrometry analysis
- Remove water, organic acids, sugars, fatty acids and pigments from sample
- Design for AOAC and other QuEChERS methods
- Available in customized forms or contents

Accessories (7)
Accessories

Special Plate for Synthesis Macromolecule (22)
Synthesis Macromolecules
For high throughput screening (HTS) applications (e.g. purification of combinatorial libraries or screening pharmaceuticals in biological fluids).It is available in the versatile 96-well format. In this format, 20 um of frit is packed into your choice of 1 ml or 2ml Array column. These are suitable for extraction of 150 – 200 uL of aqueous sample. The columns can be used individually, or assembled into the plate format. Pre-assembled plates can also be purchased. The plate can be populated with as many or as few columns as required, making this a flexible, economical format.
| AQUAMATRIX SLLE | File size: 376KB | Download |
| RUBYPRO Protein Crash | File size: 846KB | Download |
| Orpheus | File size: 3.97MB | Download |
| Panthera Deluxe SPE Cartridge | File size: 2.7MB | Download |

 Read more
Read more